The grace period related to the Poison Center Notifications (PCN) to the European Union (EU) Poison Centers ended on January 1, 2025. This means that companies must submit and successfully notify the unified format of notification dossiers before they can place their products on the EU market.
After the deadline for industrial mixtures passed on January 1, 2024, the Hungarian appointed body has been conducting reviews of the submitted PCN dossiers. The main focus of the review is to check whether the information in the dossiers complies with the requirements of the CLP Regulation. The content of the PCN notification is mostly derived from the Safety Data Sheet (SDS) of the product, especially the toxicological information, which is the entire content of Section 11 of the SDS. Therefore, to pass the review by the Hungarian appointed body, companies must prepare an SDS that is fully based on the CLP standard when submitting the PCN.
The current review focuses on the following points:
- If a component is listed in Annex VI of the CLP Regulation, the GHS classification must comply with the relevant provisions.
- The classification of mixtures must be carried out in accordance with the regulatory requirements and must not contradict the classification of the components.
- The notification to the Hungarian appointed body must be filled out in the official Hungarian language, with clear and unambiguous wording.
- The toxicological information must be clearly stated, and irrelevant information to the product should be avoided.
- The toxicological information must be consistent with the mixture classification and include relevant descriptions for the classification.
The notification requires submission of content not included in the SDS:
When submitting the PCN notification, many companies have a misconception that the information filled in the dossier is a direct copy of the SDS. This is not the case. The PCN dossier must include some information that is not found in the SDS, such as:
- The type and specifications of the product packaging, for example, bottle, 1 kg.
- If the mixture does not have a pH value, a reason must be selected, such as the mixture is insoluble in water.
- When describing the main uses of the product, it must be detailed and clear, mentioning the fields of application.
- If the mixture components are expressed as concentration ranges, they must comply with the regulatory requirements. Regarding the filling of concentration ranges, the CLP Regulation has stricter requirements for PCN submission than for SDS preparation. The relevant requirements are as follows:
When a mixture component belongs to at least one of the following hazard categories, its concentration in the mixture must be indicated by an exact percentage, in descending order of mass or volume:
- Acute toxicity, Category 1, 2, or 3
- Specific target organ toxicity – Single exposure, Category 1 or 2
- Specific target organ toxicity – Repeated exposure, Category 1 or 2
- Skin corrosion, Category 1, 1A, 1B, or 1C
- Serious eye damage, Category 1
In addition to providing exact concentrations, concentration ranges can also be submitted according to Table 1.
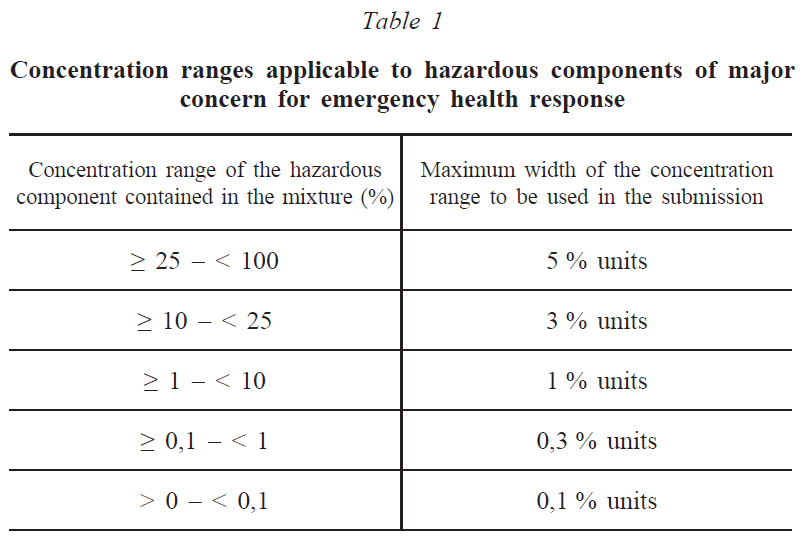
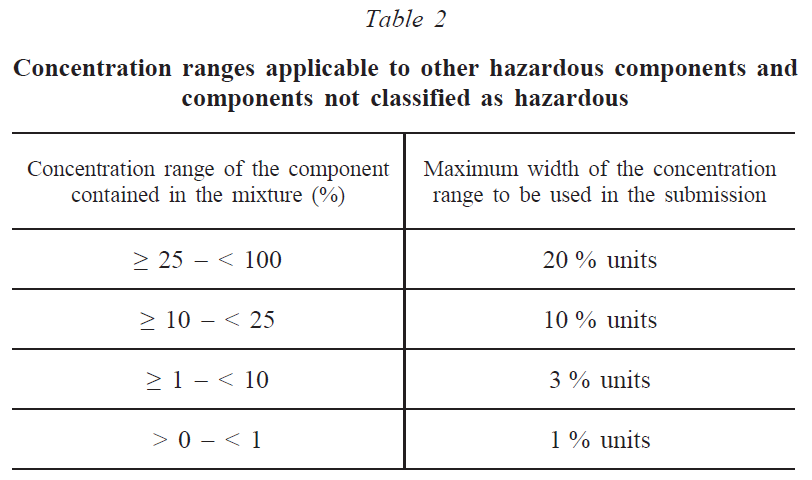
The concentration of hazardous components in the mixture that have not been classified in any of the above hazard categories and identified components that have not been classified as hazardous must be indicated by concentration ranges in descending order of mass or volume according to Table 2. Alternatively, exact concentrations can be provided.
Now that the regulation has been implemented, the appointed bodies of each member state will gradually review the submitted PCN dossiers. CIRS Group reminds you to check whether your product has an SDS that complies with the CLP standard, as this is a prerequisite for submitting the PCN. Companies that have already submitted the PCN may face a significant amount of PCN update work in the future.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information
Chemradar Guides: EU Poison Center Notification

