On March 31, 2023, the European Commission released the amendments to Annex I to Regulation (EC) No 1272/2008 as regards hazard classes and criteria for the classification, labelling and packaging of substances and mixtures (CLP), introducing several new classification criteria to identify endocrine disrupting chemicals (EDCs) and persistent and mobile substances.
The amended regulation also introduced four hazard classes for persistent and mobile substances. These are:
- persistent, bioaccumulative and toxic (PBT);
- very persistent, very bioaccumulative (vPvB);
- persistent, mobile and toxic (PMT); and
- very persistent, very mobile (vPvM).
Details are as follows:
Endocrine Disruption Chemicals
In accordance with the requirements of Part 3 under Annex I, endocrine disruptors for human health/ the environment can be allocated into one of two categories:
Hazard category | Hazard criteria for endocrine disruptors for human health | Hazard criteria for endocrine disruptors for the environment |
Category 1 | Known or presumed endocrine disruptors for human health. The classification in Category 1 shall be largely based on evidence from at least one of the following:
Such data shall provide evidence that the substance meets all the following criteria:
However, where there is information that raises serious doubt about the relevance of the adverse effects to humans, classification in Category 2 may be more appropriate. | Suspected endocrine disruptors for environment. A substance shall be classified in Category 2 where all the following criteria are fulfilled: (a) there is evidence of: i. an endocrine activity; and ii. an adverse effect in an intact organism or its offspring or future generations; (b) the evidence referred to in point (a) is not sufficiently convincing to classify the substance in Category 1; (c) there is evidence of a biologically plausible link between the endocrine activity and the adverse effect. |
Category 2 | Suspected endocrine disruptors for human health. A substance shall be classified in Category 2 where all the following criteria are fulfilled: (a) there is evidence of: i. an endocrine activity; and ii. an adverse effect in an intact organism or its offspring or future generations; (b) the evidence referred to in point (a) is not sufficiently convincing to classify the substance in Category 1; and (c) there is evidence of a biologically plausible link between the endocrine activity and the adverse effect. | Suspected endocrine disruptors for the environment. A substance shall be classified in Category 2 where all the following criteria are met: (a) there is evidence of: i. an endocrine activity; and ii. an adverse effect in an intact organism or its offspring or future generations; (b) the evidence referred to in point (a) is not sufficiently convincing to classify the substance in Category 1; and (c) there is evidence of a biologically plausible link between the endocrine activity and the adverse effect. |
Generic concentration limits of components of a mixture classified as an endocrine disruptor for human health/the environment that trigger the classification of the mixture:
A mixture shall be classified as an endocrine disruptor for human health where at least one component has been classified as a Category 1 or Category 2 endocrine disruptor for human health and is present at or above the appropriate generic concentration limit as shown in the Table below for Category 1 and Category 2, respectively.
Component classified as: | Generic concentration limits triggering the classification of a mixture as: | |
Category 1 endocrine disruptor for human health | Category 2 endocrine disruptor for human health | |
Category 1 endocrine disruptor for human health | ≥ 0.1 % | - |
Category 2 endocrine disruptor for human health | - | ≥ 1 %※ |
※ The concentration limits in this Table shall apply to solids and liquids (w/w units) as well as gases (v/v units). If a Category 2 endocrine disruptor for human health is present in the mixture as an ingredient at a concentration ≥ 0.1 % a SDS shall be available for the mixture upon request.
A mixture shall be classified as an endocrine disruptor for the environment where at least one component has been classified as a Category 1 or Category 2 endocrine disruptor for the environment and is present at or above the appropriate generic concentration limit as shown in Table below for Category 1 and Category 2, respectively.
Component classified as: | Generic concentration limits triggering the classification of a mixture as: | |
Category 1 endocrine disruptor for the environment | Category 2 endocrine disruptor for the environment | |
Category 1 endocrine disruptor for the environment | ≥ 0.1 % | - |
Category 2 endocrine disruptor for the environment | - | ≥ 1 %※ |
※ The concentration limits in this Table apply to solids and liquids (w/w units) as well as gases (v/v units). If a Category 2 endocrine disruptor for the environment is present in the mixture as an ingredient at a concentration ≥ 0.1 % a SDS shall be available for the mixture upon request.
Label Elements
Label elements of endocrine disruption for human health
Classification | Category 1 | Category 2 |
Symbol/pictogram |
|
|
Signal Word | Danger | Warning |
Hazard Statement | EUH380: May cause endocrine disruption in humans | EUH381: Suspected of causing endocrine disruption in humans |
Precautionary Statement Prevention | P201 P202 P263 P280 | P201 P202 P263 P280 |
Precautionary Statement Response | P308 + P313 | P308 + P313 |
Precautionary Statement Storage | P405 | P405 |
Precautionary Statement Disposal | P501 | P501 |
From May 1, 2025, at the latest, substances shall be labeled in accordance with the above classifications; However, substances that were placed on the market before May 1, 2025, are not required to be labeled in accordance with the above classifications until November 1, 2026.
Mixtures shall be labeled in accordance with the above classifications from May 1, 2026, at the latest; while mixtures that were placed on the market before May 1, 2026, are not required to be labeled in accordance with the above classifications until May 1, 2028.
Label elements of endocrine disruption for the environment
Classification | Category 1 | Category 2 |
Symbol/pictogram |
|
|
Signal Word | Danger | Warning |
Hazard Statement | EUH430: May cause endocrine disruption in the environment | EUH431: Suspected of causing endocrine disruption in the environment |
Precautionary Statement Prevention | P201 P202 P273 | P201 P202 P273 |
Precautionary Statement Response | P391 | P391 |
Precautionary Statement Storage | P405 | P405 |
Precautionary Statement Disposal | P501 | P501 |
From May 1, 2025, at the latest, substances shall be labeled in accordance with the above classifications. However, substances that were placed on the market before May 1, 2025, are not required to be labeled in accordance with the above classifications until November 1, 2026.
Mixtures shall be labeled in accordance with the above classifications from May 1, 2026, at the latest; while mixtures that were placed on the market before May 1, 2026, are not required to be labeled in accordance with the above classifications until May 1, 2028.
PBT or vPvB Properties
Classification criteria for PBT and vPvB substances:
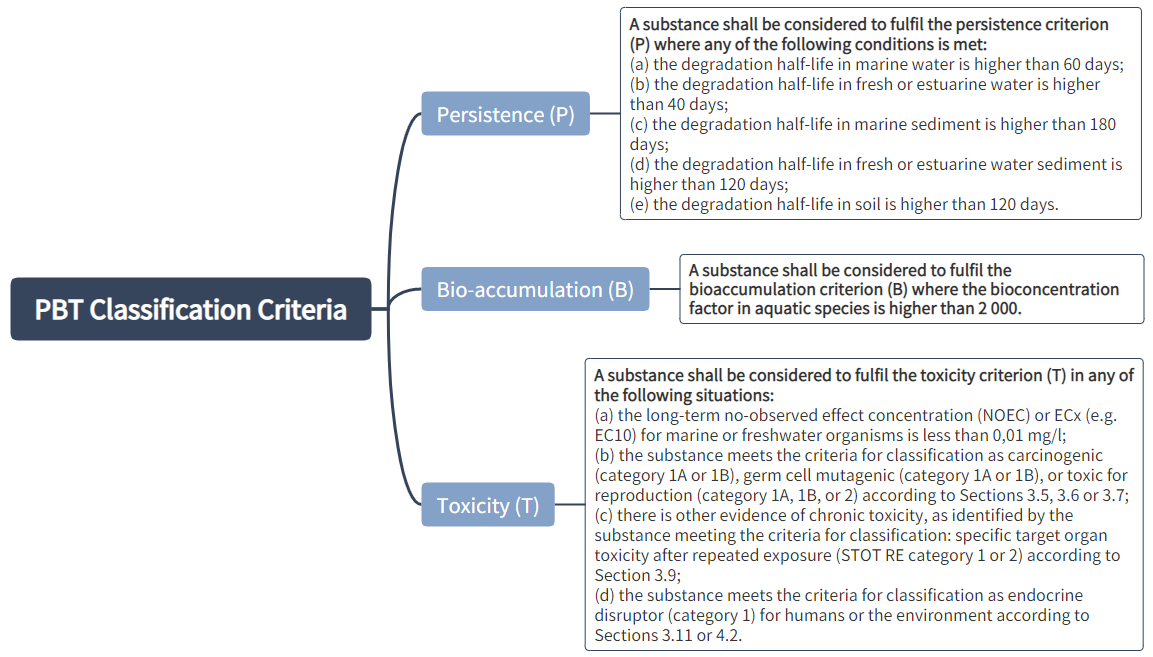
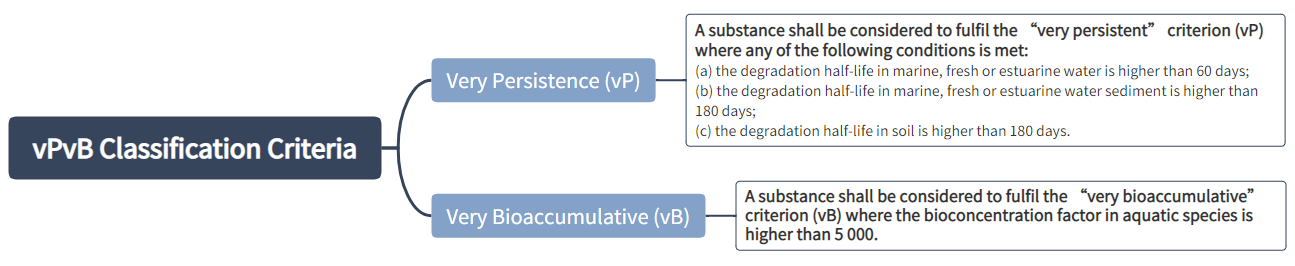
A mixture shall be classified respectively as a PBT or vPvB when at least one component contained in the mixture has been classified respectively as a PBT or vPvB and is present at or above 0.1 % (weight/weight).
Label Elements
Label elements for PBT and vPvB properties
| PBT | vPvB |
Symbol/pictogram |
|
|
Signal Word | Danger | Danger |
Hazard Statement | EUH440: Accumulates in the environment and living organisms including humans | EUH441: Strongly accumulates in the environment and living organisms including humans |
Precautionary Statement Prevention | P201 P202 P273 | P201 P202 P273 |
Precautionary Statement Response | P391 | P391 |
Precautionary Statement Storage | P501 | P501 |
Classification criteria for PMT and vPvM substances:
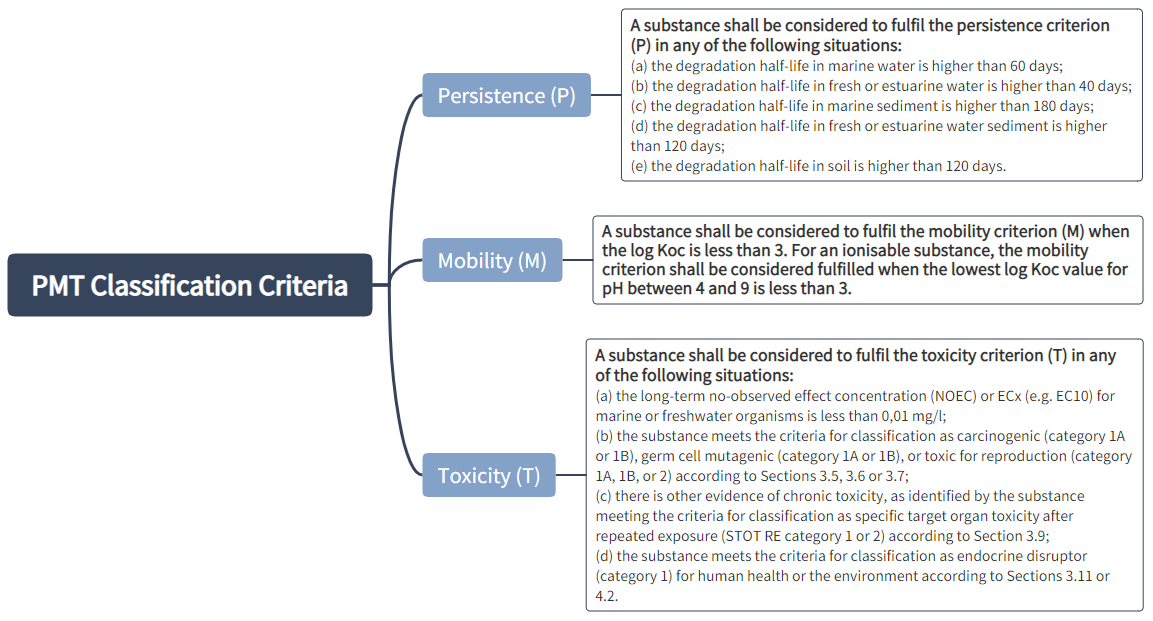
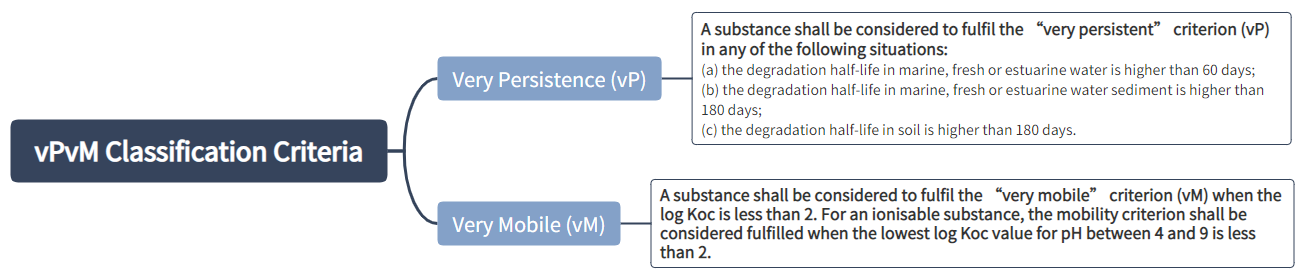
A mixture shall be classified as a PMT or vPvM where at least one of its components has been classified as a PMT or vPvM and is present at or above 0.1 % (weight/weight).
Label Elements
Label elements for PMT and vPvM properties
| PMT | vPvM |
Symbol/pictogram |
|
|
Signal Word | Danger | Danger |
Hazard Statement | EUH450: Can cause long-lasting and diffuse contamination of water resources | EUH451: Can cause very long-lasting and diffuse contamination of water resources |
Precautionary Statement Prevention | P201 P202 P273 | P201 P202 P273卡 |
Precautionary Statement Response | P391 | P391 |
Precautionary Statement Storage | P501 | P501 |
CIRS Comments
There is no doubt that related enterprises may face various challenges as new hazard classifications have been introduced. Manufacturers of related substances should re-evaluate the hazards of substances within the transitional period. The legislative framework of several legislations, including chemicals, biocides, pesticides, toys as well as medical devices may have been affected.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information
EU Vigorously Advances Animal Testing Alternatives, OECD 416 and UDS Removed

