On January 16, 2025, the UK notified the World Trade Organization (WTO) of the draft Cosmetic Products (Restriction of Chemical Substances) Regulations 2025, proposing amendments to Annex III of Regulation (EC) No. 1223/2009 on cosmetic products to restrict the use of Methyl Salicylate in certain cosmetic formulations. The regulation is scheduled to be adopted on April 30, 2025, and will officially come into effect on September 30, 2025.
Methyl Salicylate has been classified as a category 2 CMR substance under the EU Classification, Labelling, and Packaging (CLP) Regulation. Based on the opinion of the Scientific Advisory Group on Consumer Safety (SAG-CS), Annex III of the regulation will be amended to allow the use of Methyl Salicylate in cosmetic products, provided that specific restriction conditions are met.
Amendment Details
INCI Name | CAS No. | EC No. | Product Type | Methyl Salicylate Usage Restrictions |
Methyl Salicylate | 119-36-8 | 204-317-7 | (A) Rinse off skin & hair products (except hand wash products) (B) Hand wash products (C) Leave on skin products (except face makeup, spray/aerosol body lotion, spray/aerosol deodorant, and hydroalcoholic-based fragrances) and hair products (non-aerosol) (D) Lipsticks & lip balm (E) Face makeup products (F) Eye makeup products & makeup remover (G) Toothpaste (H) Mouthwash (I) Mouth spray (J) Hydroalcoholic-based fragrances (K) Deodorant spray/aerosol products (L) Hair products (spray/aerosol) (M) Body Lotion Spray | (A) (1) 0.02% (products intended for children 0.5-1 years) (2) 0.06% (products intended for children above 1 year and adults) (B) (1) 0.02% (products intended for children 0.5-1 years) (2) 0.6% (products intended for children above 1 year and adults) (C) (1) 0.02% (products intended for children 0.5-1 years) (2) 0.06% (products intended for children above 1 year and adults) (D) (1) 0.02% (products intended for children 0.5-1 years) (2) 0.03% (products intended for children above 1 year and adults) (E) 0.05% (F) 0.002% (G) 2.5% (H) (1) 0.1% (products intended for children 6 to 10 years) (2) 0.4% (products intended for children above 10 years and adults) (I) 0.65% (J) 0.6% (K) 0.003% (L) 0.009% (M) 0.04% |
Transitional arrangements for cosmetic products containing Methyl Salicylate: Products already placed on the market before September 30, 2025, can continue to be made available on the market until March 31, 2026.
Interpretation of Methyl Salicylate
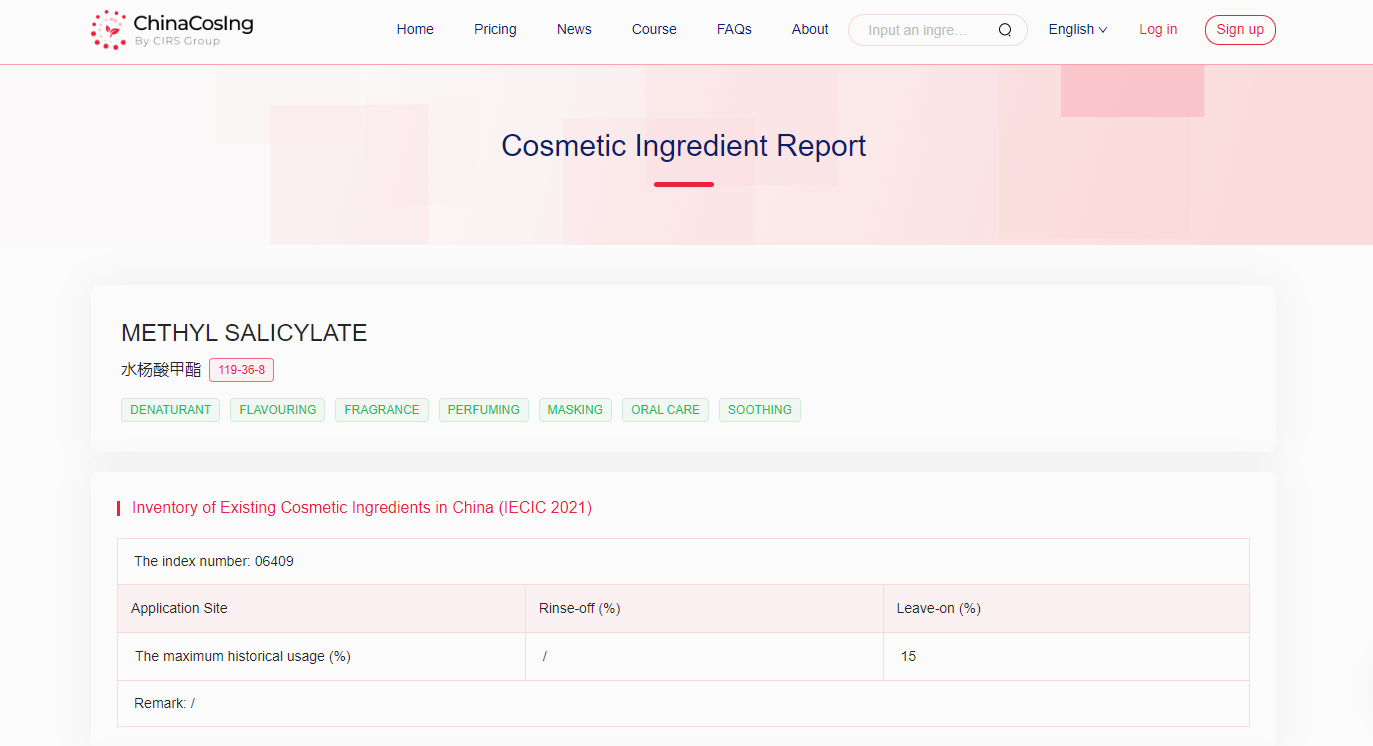
[Regulatory Requirements in China] The Chinese Cosmetic Ingredient Regulatory Database (ChinaCosIng), independently developed by CIRS Group, indicates that Methyl Salicylate (CAS No. 119-36-8) can be used as denaturant, flavouring, fragrance, perfuming, masking, oral care or smoothing in cosmetics. Methyl Salicylate has been included in the “Inventory of Existing Cosmetic Ingredients in China (IECIC) (2021 edition)”. The maximum historical usage in leave-on products is 15%.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information
https://members.wto.org/crnattachments/2025/TBT/GBR/25_00683_00_e.pdf

