According to the "Regulations on the Supervision and Administration of Medical Devices" (Order No. 739), Class I medical devices are subject to product filing management, and clinical trials are not required, but clinical evaluation data need to be submitted; when Class II and III medical devices are registered , clinical trials should be conducted (except for medical devices in the list of medical devices exempt from clinical trials); medical devices exempt from clinical trials must still provide clinical evaluation data when applying for registration.
Service Process:
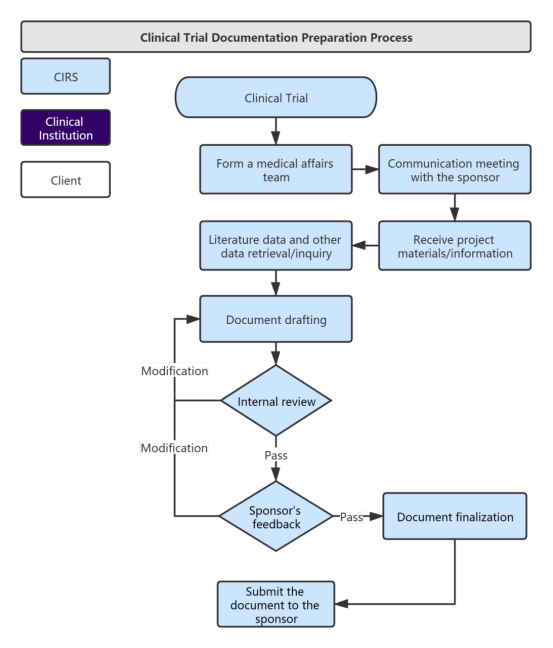
Our Services and Time Distribution:
Clinical trial protocol 20 working days
Informed consent form 5 working days
Clinical trial summary report 20 working days
Clinical evaluation report 20-120 working days
Investigator’s brochure 10 working days