According to the "Regulations on the Supervision and Administration of Medical Devices" (Order No. 739), Class I medical devices are subject to product filing management, and clinical trials are not required, but clinical evaluation data need to be submitted; when Class II and III medical devices are registered , clinical trials should be conducted (except for medical devices in the list of medical devices exempt from clinical trials); medical devices exempt from clinical trials must still provide clinical evaluation data when applying for registration.
Service Process:
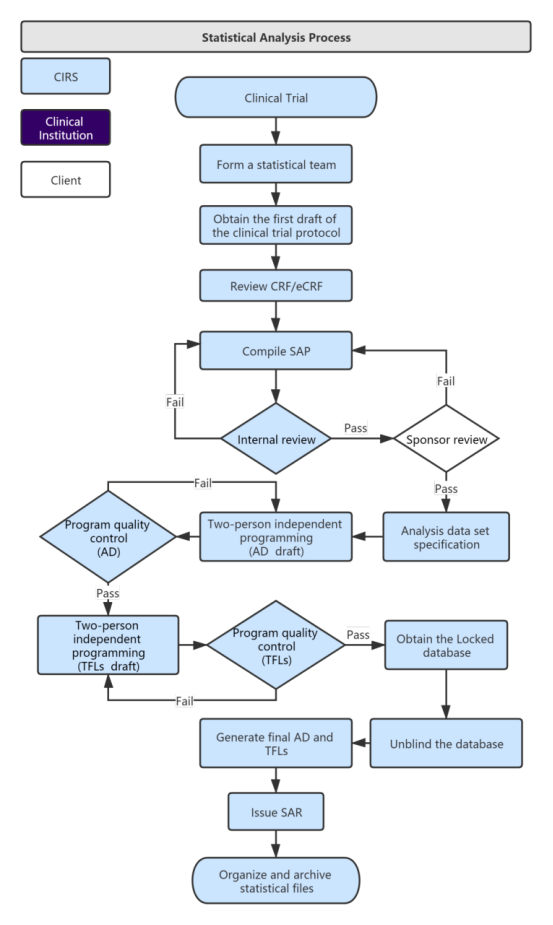
Our Services and Time Distribution:
- Randomization plan, random table template(first draft) 3 working days after the plan is finalized
- Statistical analysis plan (first draft) 7 working days after the protocol is finalized
- SAS programming 25 working days after the database is built
- Statistical analysis report (first draft) 10 working days