Infant formula milk powder has been highly concerned by government and society, and the quality control and supervision by Chinese government become stricter in recent years. The Food Safety Law of China (2015 version) stipulates that the formula of infant milk powder should be registered under CFDA.
Since January 1 2018, all infant formula milk powder products produced in China or imported to China shall get the registration certificate with CFDA, and indicate the registration number on the label and instruction book (Products that produced before January 1, 2018 can still be on market till expire date).
Classification of Infant Formula Milk Powder
The infant formula milk powder refers to the powder product that mainly made from milk, milk powder, milk protein products, etc., added with appropriate vitamins, minerals, etc., produced by physical method, and suitable for normal infants. There are 3 classifications of infant formula milk powder in China, namely,
Infant formula milk powder (0-6 months, stage 1)
Older infant formula milk powder (6-12 months, stage 2)
Young children formula milk powder (13-36 months, stage 3)
Laws, Regulations and National Standards
Laws and Regulations | Issued Date | Implemented Date |
|---|---|---|
Food Safety Law of The People’s Republic of China (2015 version) | 2015.04.24 | 2015.10.01 |
Administrative Measure on Product Formula Registration of Infant Formula Milk Powder | 2016.06.08 | 2016.10.01 |
Dossier and Requirements of Infant Formula Milk Powder Formula Registration (trial) (2017 revision) | 2017.05.23 | 2017.05.23 |
Technical Guidelines on Labelling of Infant Formula Milk Powder Formula Registration (trial) | 2017.05.24 | 2017.05.24 |
On-site Inspection Key Points and Judgment Principles on Infant Formula Milk Powder Formula Registration (trial) | 2016.11.16 | 2016.11.16 |
GB 13432-2013 General Rules for Labeling of Prepackaged Food for Special Dietary Uses | 2013.12.26 | 2015.07.01 |
GB 10765-2010 Infant Formula | 2010.03.26 | 2011.04.01 |
GB 10767-2010 Older Infants and Young Children Formula | 2010.03.26 | 2011.04.01 |
(&mldr&mldr&mldr&mldr&mldr&mldr&mldr.) | - | - |
Applicant Qualification
No matter for domestic or imported infant formula milk powder products, the applicants shall be actual manufacturers who have production capacity
Note: The overseas applicants shall get the overseas manufacturer registration under Certification and Accreditation Administration of China (CNCA) as well, before importing the products to China.
Registration Quantities for One Company
There are at most 3 series and 9 kinds of formulas for one company in principle (a series of formulas include infant formula for stage 1, infant formula for stage 2, and infant formula for stage 3);
There should be significant differences among three formulas for the same stage;
One wholly-owned subsidiary who has got the formula registration license and production license is allowed to produce the registered product from another wholly-owned subsidiary in the same group company.
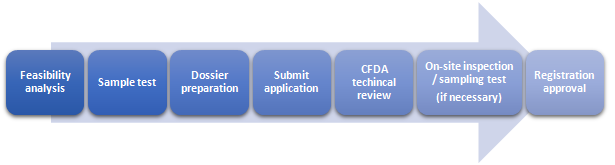
Registration Procedures
Dossier Requirements
Application form
Subject qualification certifications of the applicant
Raw material quality and safety standards
Product formula
Product formula R&D report
Production process description
Product test reports
Evidentiary materials on production capacity, R&D capacity and inspection capacity
Samples of label and specification and related certificates
Test Items
Test items | Batch amount | Sample requirement |
|---|---|---|
Tests of all items listed in GB 10765 or 10767 and relevant laws and regulations | 3 batches (at least one batch from commercial production line) | Finished product |
Other tests if necessary | - | - |
Our Services
CIRS is providing one-stop services of China food regulatory compliance. For infant formula, we offer the following services:
1. Training on Infant Food Regulation
2. Infant Food Regulation Update Monitoring
3. Infant Formula Milk Powder Registration
4. Single Technology Services
Infant Formula Milk Powder Formula Review
Test Arrange and Monitoring
Chinese Label Compliance Services
Other Customized Services