According to data from the SAMR's Special Food Information Inquiry Platform, 12 new foods for special medical purposes were added on December 28, 2024. This includes two imported products and ten domestic products, involving nine companies. The product categories cover:
- complete nutrition formula foods;
- protein (amino acid) components;
- carbohydrate components;
- electrolyte formulas;
- liquid formulas; and
- formulas for premature/low birth weight infants.
Table 1. Information on the 12 Foods for Special Medical Purposes
Origin | Registration Number | Product Name | Company Name | Expiration Date | Product Type | Form |
Domestic | National Food Registration Number TY20240054 | Shangheng Special Medical Purpose Electrolyte Formula Food | Guangdong Yuewei Biological Technology Co., Ltd. | 2029/12/28 | Electrolyte Formula | Liquid |
Domestic | National Food Registration Number TY20240055 | Weiyuan Jintai Special Medical Purpose Protein Component Formula Food | Yabao Pharmaceutical Group Co., Ltd. | 2029/12/28 | Protein (Amino Acids) Component | Powder |
Domestic | National Food Registration Number TY20240056 | Hengyi Fuyuan Special Medical Purpose Liquid Formula Powder | Wuxi Hengyi Health Technology Co., Ltd. | 2029/12/28 | Liquid Formula | Powder |
Domestic | National Food Registration Number TY20240057 | Baiquan Futong Special Medical Purpose Complete Nutrition Formula Liquid | Kunyu Health Pharmaceutical Jiangsu Co., Ltd. | 2029/12/28 | Complete Nutrition Formula Food | Liquid |
Domestic | National Food Registration Number TY20240058 | Xinyi Jiatai Special Medical Purpose Complete Nutrition Formula Food | Chenxin Pharmaceutical Co., Ltd. | 2029/12/28 | Complete Nutrition Formula Food | Liquid |
Domestic | National Food Registration Number TY20240059 | Baiquan Quanheng Special Medical Purpose Complete Nutrition Formula Liquid | Kunyu Health Pharmaceutical Jiangsu Co., Ltd. | 2029/12/28 | Complete Nutrition Formula Food | Liquid |
Domestic | National Food Registration Number TY20240060 | Beilejia Special Medical Purpose Electrolyte Formula Food | Shandong Lihao Special Medical Purpose Formula Food Co., Ltd. | 2029/12/28 | Electrolyte Formula | Powder |
Domestic | National Food Registration Number TY20240061 | Gaobaili Special Medical Purpose Carbohydrate Component Formula Food | Shandong Lihao Special Medical Purpose Formula Food Co., Ltd. | 2029/12/28 | Carbohydrate Component | Powder |
Domestic | National Food Registration Number TY20240062 | Weite Yuan Special Medical Purpose Protein Component Formula Food | Tianjin Hezhi Guangping Pharmaceutical Co., Ltd. | 2029/12/28 | Protein (Amino Acids) Component | Powder |
Domestic | National Food Registration Number TY20240063 | Nengquanxin Special Medical Purpose Protein Component Formula Food | Nutricia Pharmaceutical (Wuxi) Co., Ltd. | 2029/12/28 | Protein (Amino Acids) Component | Liquid |
Imported | National Food Registration Number TY20245002 | Qifu Yuzao Special Medical Purpose Preterm/Low Birth Weight Infant Formula Food | Wyeth Nutrition (Singapore) Private Limited | 2029/12/28 | Preterm/Low Birth Weight Infant Formula | Powder |
Imported | National Food Registration Number TY20245003 | Xiaojiashan Xinneng Special Medical Purpose Complete Nutrition Formula Food | Wyeth Nutrition (Singapore) Private Limited | 2029/12/28 | Complete Nutrition Formula Food | Powder |
Related Link
As of January 7, 2025, the number of FSMPs approved in 2024 (with National Food Registration Numbers starting with "TY2024XXXX") has increased to 66, bringing the total number of approved FSMPs in China to 230.
CIRS Group has conducted a detailed statistical summary and analysis of the 66 foods for special medical purposes approved in 2024 from multiple perspectives as follows.
1. General Situation of FSMP Approvals in 2024
In 2024, of all the approved FSMPs, 63 were domestic products, accounting for 95%; and three were imported products, accounting for 5%. The number of domestic product registrations far exceeded that of imported products. In terms of product dosage forms, powder products were the most common, with a total of 45, accounting for 68%; liquid products numbered 20, accounting for 30%, and there was also one0 product with a granular dosage form.
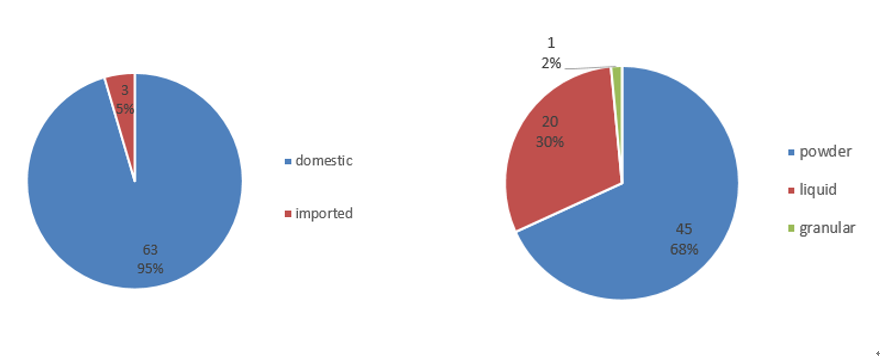
Figure 1. General Situation of FSMP Registration Approvals in 2024
2. The Number of FSMP Registrations Approved in 2024
The registration approvals for FSMPs in 2024 are shown in Figure 2. It shows that with the exception of three months, several products were approved every month throughout the year. This indicates that the enthusiasm for registering FSMPs remains high. At the same time, the General Administration has significantly improved the efficiency of review and approval, supporting the development of the industry.
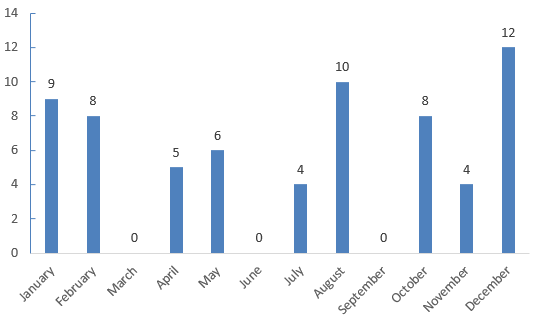
Figure 2. FSMP Registrations Approved in Each Month 2024
3. The Number of FSMP Registrations Approved in 2024 (by Product Category)
As shown in Figure 3 below, among the FSMPs approved for registration in 2024, the number of complete nutrition formula foods was the highest, with a total of 21, accounting for 32% of the total; followed by protein (amino acid) components – 12 – accounting for 18% of the total.
At the same time, some product categories did not have new products approved in 2024, such as extensively hydrolyzed milk protein formulas, amino acid formulas, amino acid metabolism disorder formulas, and specific complete nutrition formulas.
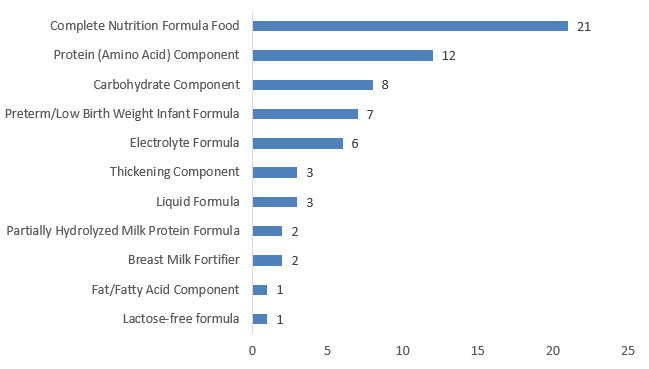
Figure 3. Number of Registrations Approved for Different Categories of Special Medical Purpose Foods in 2024
4. The Number of FSMP Registrations Approved in 2024 (by Targeted Population)
As shown in Figure 4 below, among the FSMPs approved for registration in 2024, the largest number of products were intended for people aged ten and above, totaling 40, which accounted for 61% of the total. The next were products intended for infants aged 0-12 months, totaling 12, which accounted for 18% of the total. Additionally, there were eight products intended for children aged 1-10 years, and six products intended for people aged one and above.
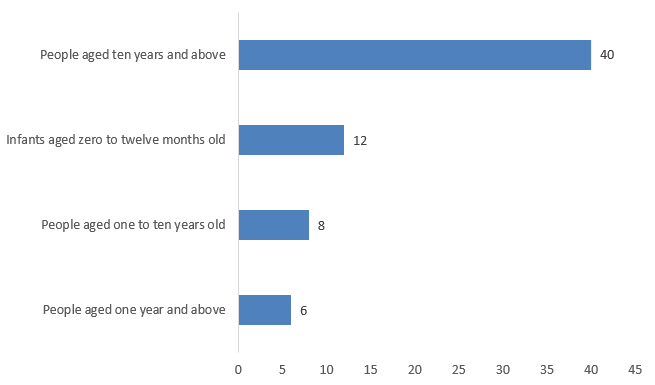
Figure 4. The Number of Approved FSMP Registrations for Different Ages in 2024
5. The Number of FSMP Registrations Approved in 2024 (by Province/Region)
The 66 FSMPs approved for registration in 2024 came from 17 different provinces and regions. Jiangsu, Shandong, and Heilongjiang provinces ranked top three with 21, 10, and 6 products respectively, accounting for 32%, 15%, and 9% of the total approved number. For specific provinces and regions along with their data, see Figure 5 below.
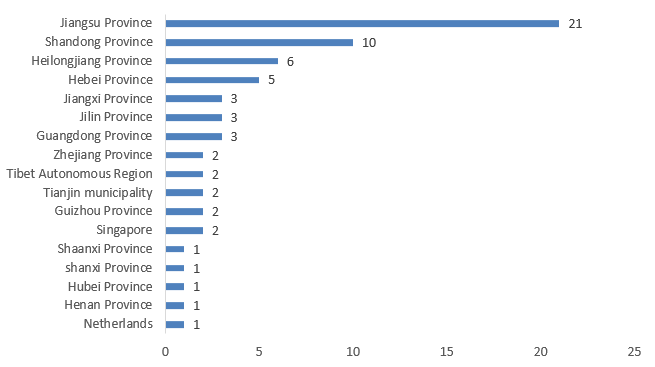
Figure 5. Number of Registrations Approved for Special Medical Purpose Foods by Province/Region in 2024
6. The Number of FSMP Registrations Approved in 2024 (by Applicant Unit)
The 66 FSMPs approved for registration in 2024 involved 36 applicant units. As shown in Figure 6 below, Kunyu Health Pharmaceutical Jiangsu Co., Ltd. obtained five special medical purpose food approvals in 2024, ranking first. Additionally, three companies each obtained four special medical purpose food approvals, belonging to the second tier. Two companies each obtained three special medical purpose food approvals, belonging to the third tier. The remaining companies each obtained two or one special medical purpose food approvals.
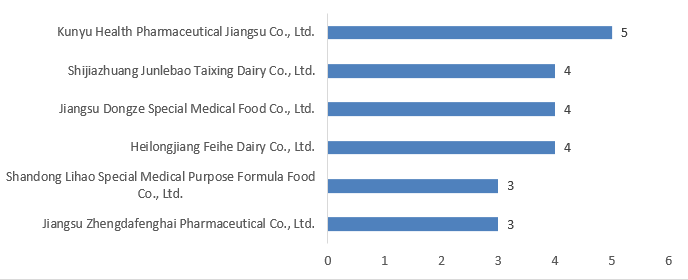
Figure 6. Top Six Companies in Number of FSMP Registrations Approved in 2024
CIRS Analysis
From the statistical analysis, it can be observed that the number of approvals for special medical purpose foods in 2024 continued the high growth trend seen in 2023, with a total of 70 products registered and approved in 2023. Such data indicates that the development and registration process for special medical purpose foods is becoming more efficient.
In terms of policy support, in 2024, SAMR issued a series of guidelines and procedures to optimize the registration process for special medical purpose foods. On July 19, 2024, SAMR released the “Special Medical Purpose Electrolyte Formula Food Registration Guide”, “Special Medical Purpose Carbohydrate Component Formula Food Registration Guide” and “Special Medical Purpose Protein Component Formula Food Registration Guide”. These guides optimize the registration application materials for the three types of component products, generally eliminating the need for on-site registration inspections and sampling tests, which helps enterprises reduce research and development costs, shorten registration time, and improve the efficiency of review and approval. Additionally, on October 22, 2024, SAMR issued the “Special Medical Purpose Formula Food Registration Priority Review and Approval Work Procedures”, which shorten the review period, prioritizes on-site inspections and sampling tests, and prioritizes clinical trial site inspections for new types of special medical purpose foods that are urgently needed clinically and have not yet been approved, such as those for rare diseases.
CIRS Group recommends that enterprises should conduct thorough preparations before applying for the registration of special medical purpose foods. This includes conducting market analysis to understand market demand, literature research to substantiate the scientific basis of the formula, and factory audits to ensure compliance in the trial production process. CIRS Group provides comprehensive technical services for the registration of special medical purpose formula foods and welcomes enterprises in need to contact us at any time!
Notes:
1. Data Source: Special Food Information Inquiry Platform.
2. There may be a delay in the release of data from the Special Food Information Inquiry Platform. The data in this article is for reference only, and the actual situation should be based on official announcements.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

