As of the end of December 2024, the National Health Commission of the People’s Republic of China (NHC) issued four announcements (No. 2 of 2024, No. 3 of 2024, No. 5 of 2024, No. 6 of 2024) regarding “Three New Foods” (new food raw materials, new food additives and food-related products). A total of 54 Three New Foods have been approved in 2024, 30 of which are new food additives, including those with expanded scope.
Related Link
Analysis on the Application and Approval of New Food Raw Materials (Novel Food) in 2024
CIRS Group has summarized the acceptance and approval status of new food additives in China in 2024 as follows:
1. Overview of new food additives accepted, issued for public comments and approved in 2024
In 2024, NHC accepted the application of 90 new food additives, including new types and those with expanded use and scope. The China National Center for Food Safety Risk Assessment (CFSA) issued a total of 41 new food additives for public comments; moreover, the HNC approved 30 new food additives, including the ones with expanded scope.
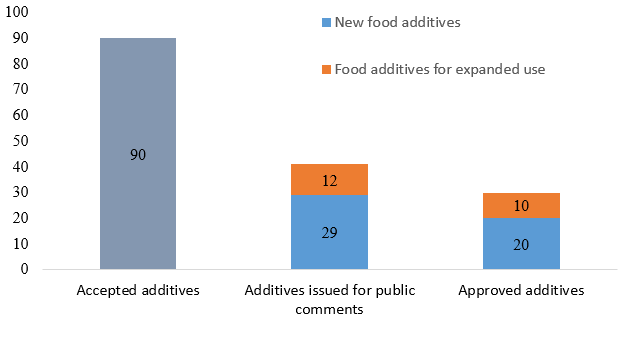
Figure 1. Overview of new food additive applications in 2024
2. List of accepted new food additives in 2024 (90 types)
In 2024, NHC accepted the application of 90 new food additives, including those with expanded scope, with the acceptance codes of both domestic and imported products presented as “卫食添新申字”. Detailed information is shown in the Table below, substances marked in red indicate that a “Decision of Administrative Disapproval” has been issued, totaling 20 substances. Substances marked in blue indicate that a “Notice of Review Comments” has been issued, totaling 6 substances. The remaining substances are either under extended review or in the public consultation.
No. | Acceptance date | Acceptance code | Name of new food additives |
|---|---|---|---|
1 | 2024-01-05 | 卫食添新申字(2024)第0001号 | Marigold yellow |
2 | 2024-01-11 | 卫食添新申字(2024)第0002号 | 2'-Fucosyllactose, 2’FL |
3 | 2024-01-12 | 卫食添新申字(2024)第0003号 | Lactose-N-tetraose |
4 | 2024-01-16 | 卫食添新申字(2024)第0004号 | L-Alanine |
5 | 2024-01-16 | 卫食添新申字(2024)第0005号 | 2'-Fucosyllactose |
6 | 2024-01-16 | 卫食添新申字(2024)第0006号 | Xanthan gum |
7 | 2024-01-17 | 卫食添新申字(2024)第0007号 | Lipase |
8 | 2024-01-18 | 卫食添新申字(2024)第0008号 | Selenium-enriched yeast |
9 | 2024-01-18 | 卫食添新申字(2024)第0009号 | 2'-Fucosyllactose, 2’FL |
10 | 2024-01-18 | 卫食添新申字(2024)第0010号 | Chitosan |
11 | 2024-01-22 | 卫食添新申字(2024)第0011号 | Anethole |
12 | 2024-01-22 | 卫食添新申字(2024)第0012号 | Citral |
13 | 2024-01-22 | 卫食添新申字(2024)第0013号 | Hydroxycitronellal |
14 | 2024-01-23 | 卫食添新申字(2024)第0014号 | 3'-Sialyllactose sodium salt |
15 | 2024-01-24 | 卫食添新申字(2024)第0015号 | Xylanase |
16 | 2024-01-24 | 卫食添新申字(2024)第0016号 | D-Allulose-3-epimerase |
17 | 2024-01-24 | 卫食添新申字(2024)第0017号 | β-Alanine |
18 | 2024-01-25 | 卫食添新申字(2024)第0018号 | Glucose oxidase |
19 | 2024-01-26 | 卫食添新申字(2024)第0019号 | Polyglycerol ricinoleate |
20 | 2024-01-30 | 卫食添新申字(2024)第0020号 | Peroxidase |
21 | 2024-02-06 | 卫食添新申字(2024)第0021号 | Glucoamylase |
22 | 2024-02-19 | 卫食添新申字(2024)第0022号 | Rebaudioside AM (enzyme conversion method) |
23 | 2024-02-19 | 卫食添新申字(2024)第0023号 | Rebaudioside M (enzyme conversion method) |
24 | 2024-03-04 | 卫食添新申字(2024)第0024号 | Rosemary extract |
25 | 2024-03-14 | 卫食添新申字(2024)第0025号 | Sodium ferrous citrate |
26 | 2024-03-18 | 卫食添新申字(2024)第0026号 | Magnesium oxide |
27 | 2024-03-18 | 卫食添新申字(2024)第0027号 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone |
28 | 2024-03-19 | 卫食添新申字(2024)第0028号 | β-Alanine |
29 | 2024-03-20 | 卫食添新申字(2024)第0029号 | Lactose-N-tetraose |
30 | 2024-03-20 | 卫食添新申字(2024)第0030号 | Lactose-N-neotetraose |
31 | 2024-03-21 | 卫食添新申字(2024)第0031号 | Steviol glycosides |
32 | 2024-03-21 | 卫食添新申字(2024)第0032号 | Serine protease |
33 | 2024-03-22 | 卫食添新申字(2024)第0033号 | Antarcticine (Antarctic red pigment) |
34 | 2024-03-29 | 卫食添新申字(2024)第0034号 | 6'-Sialyllactose sodium salt |
35 | 2024-03-29 | 卫食添新申字(2024)第0035号 | Glucose oxidase |
36 | 2024-03-29 | 卫食添新申字(2024)第0036号 | Xylanase |
37 | 2024-03-29 | 卫食添新申字(2024)第0037号 | Catalase |
38 | 2024-04-17 | 卫食添新申字(2024)第0038号 | Recombinant human lactoferrin |
39 | 2024-04-28 | 卫食添新申字(2024)第0039号 | Fructooligosaccharides |
40 | 2024-04-30 | 卫食添新申字(2024)第0040号 | Steviol glycosides |
41 | 2024-05-07 | 卫食添新申字(2024)第0041号 | Aminopeptidase |
42 | 2024-05-07 | 卫食添新申字(2024)第0042号 | 2'-Fucosyllactose, 2’FL |
43 | 2024-05-10 | 卫食添新申字(2024)第0043号 | Hydroxycitronellal |
44 | 2024-05-10 | 卫食添新申字(2024)第0044号 | Anethole |
45 | 2024-05-13 | 卫食添新申字(2024)第0045号 | Sodium cyclamate |
46 | 2024-05-14 | 卫食添新申字(2024)第0046号 | 3-Fucosyllactose |
47 | 2024-05-17 | 卫食添新申字(2024)第0047号 | 3'-Sialyllactose sodium salt |
48 | 2024-05-20 | 卫食添新申字(2024)第0048号 | Glutaminase |
49 | 2024-05-20 | 卫食添新申字(2024)第0049号 | 2'-Fucosyllactose, 2’FL |
50 | 2024-05-21 | 卫食添新申字(2024)第0050号 | Lactose-N-tetraose |
51 | 2024-05-21 | 卫食添新申字(2024)第0051号 | Lipase |
52 | 2024-05-21 | 卫食添新申字(2024)第0052号 | β-Glucosidase |
53 | 2024-05-24 | 卫食添新申字(2024)第0053号 | Curdlan gum |
54 | 2024-06-14 | 卫食添新申字(2024)第0054号 | Curdlan gum |
55 | 2024-06-18 | 卫食添新申字(2024)第0055号 | Sucralose |
56 | 2024-06-24 | 卫食添新申字(2024)第0056号 | Tremella fuciformis sugar esters |
57 | 2024-07-05 | 卫食添新申字(2024)第0057号 | Steviol glycosides (enzyme conversion method) |
58 | 2024-07-17 | 卫食添新申字(2024)第0058号 | Nisin |
59 | 2024-07-19 | 卫食添新申字(2024)第0059号 | Hydrogen peroxide |
60 | 2024-07-29 | 卫食添新申字(2024)第0060号 | Sodium erythorbate |
61 | 2024-07-30 | 卫食添新申字(2024)第0061号 | Acesulfame potassium |
62 | 2024-08-01 | 卫食添新申字(2024)第0062号 | 2-Methyl-1-(2-(5-(p-tolyl)-1H-imidazol-2-yl)piperidin-1-yl)butan-1-one |
63 | 2024-08-02 | 卫食添新申字(2024)第0063号 | 2'-Fucosyllactose, 2’FL |
64 | 2024-08-08 | 卫食添新申字(2024)第0064号 | Lactose-N-tetraose |
65 | 2024-08-08 | 卫食添新申字(2024)第0065号 | Branching enzyme |
66 | 2024-08-08 | 卫食添新申字(2024)第0066号 | Glucoamylase |
67 | 2024-08-15 | 卫食添新申字(2024)第0067号 | Lactose-N-neotetraose |
68 | 2024-08-19 | 卫食添新申字(2024)第0068号 | 3'-Sialyllactose sodium salt |
69 | 2024-08-20 | 卫食添新申字(2024)第0069号 | Magnesium oxide |
70 | 2024-08-20 | 卫食添新申字(2024)第0070号 | L-Alanine |
71 | 2024-08-21 | 卫食添新申字(2024)第0071号 | Deaminase |
72 | 2024-08-26 | 卫食添新申字(2024)第0072号 | Isoamylase |
73 | 2024-09-04 | 卫食添新申字(2024)第0073号 | Propylene glycol |
74 | 2024-09-13 | 卫食添新申字(2024)第0074号 | Galactooligosaccharides |
75 | 2024-09-19 | 卫食添新申字(2024)第0075号 | Fructosyltransferase |
76 | 2024-10-12 | 卫食添新申字(2024)第0076号 | Deacetylated chitin (also known as Chitosan) |
77 | 2024-10-15 | 卫食添新申字(2024)第0077号 | 2'-Fucosyllactose, 2’FL |
78 | 2024-10-16 | 卫食添新申字(2024)第0078号 | Curdlan gum |
79 | 2024-10-22 | 卫食添新申字(2024)第0079号 | Carbon dioxide |
80 | 2024-10-22 | 卫食添新申字(2024)第0080号 | L-Selenomethylselenocysteine |
81 | 2024-10-24 | 卫食添新申字(2024)第0081号 | Ascorbyl palmitate (enzymatic method) |
82 | 2024-11-05 | 卫食添新申字(2024)第0082号 | 2'-Sialyllactose sodium salt |
83 | 2024-11-12 | 卫食添新申字(2024)第0083号 | 6'-Sialyllactose sodium salt |
84 | 2024-11-13 | 卫食添新申字(2024)第0084号 | D-Allulose-3-epimerase |
85 | 2024-11-13 | 卫食添新申字(2024)第0085号 | 3-Fucosyllactose |
86 | 2024-11-20 | 卫食添新申字(2024)第0086号 | 2'-Fucosyllactose |
87 | 2024-11-28 | 卫食添新申字(2024)第0087号 | Deaminase |
88 | 2024-12-04 | 卫食添新申字(2024)第0088号 | 2'-Fucosyllactose, 2’FL |
89 | 2024-12-10 | 卫食添新申字(2024)第0089号 | 3-Fucosyllactose |
90 | 2024-12-11 | 卫食添新申字(2024)第0090号 | Lactose-N-tetraose |
3. List of new food additives that have passed the technical review and were issued for public comments in 2024 (41 types)
As of the end of December 2024, 41 new food additives have passed the technical review by the review committee of China CFSA and were issued for public comments, including 9 new food enzymes, 3 new food additives, 13 new nutrition enhancer, 8 food additives with expanded use and 1food processing aids with expanded use.
1) List of new nutrition enhancer issued for public comments (13 types)
No. | Name | Applicable scope | Maximum levels | Production strain information | Approval status (By the end of December 2024) |
|---|---|---|---|---|---|
1 | 2’-fucosyllactose, 2’-FL | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.7-2.4 g/L (count as the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli BL21(DE3); Donor: E. coli O126a | 1) Issued for public comments on March 13, 2024; 2) Officially approved on October 7, 2023 according to No. 3 of 2024. |
2 | Source: E. coli K-12 GI724; Donor: Bacteroides vulgatusa | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | |||
Source: E. coli K-12 MG1655; Donor: Helicobacter spp.a | Issued for public comments on May 10, 2024; | ||||
3 | Source: E. coli BL21(DE3); Donor: Bacteroides fragilisa | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | |||
Source: E. coli BL21(DE3) Donor: Escherichia spp.a | |||||
4 | Source: E. coli BL21(DE3) Donor: Helicobacter spp.a | Issued for public comments on August 14, 2024; | |||
Source: E. coli W; Donor: Helicobacter spp.a | |||||
a is the donor for α-1,2-fucosyltransferase. | |||||
5 | 3-fucosyllactose, 3-FL | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.25-0.9 g/L (count as the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli BL21(DE3); Donor: Bacteroides fragilisa | Issued for public comments on September 26, 2024; |
6 | 3’-fucosyllactose, 3’-FL | 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.11-0.24 g/L (count as 3’-FL, the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli W NEO3 Donor: Saccharomyces cerevisiaea, Synechocystis spb, Rhodobacter capsulatusc, Pasteurellamultocidad, Neisseria lactamicae | Issued for public comments on March 13, 2024; |
Among them, a is the donor for glucosamine-6-phosphate N-acetyltransferase; b is the donor for N-acetylglucosamine-2-epimerase; c is the donor for N-acetylneuraminic acid synthase; d is the donor for cytidine-5'-monophosphate-N-acetylneuraminic acid synthase; e is the donor for α-2,3-sialyltransferase. | |||||
7 | 3’-fucosyllactose, 3’-FL | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.06-0.28 g/L (count as the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli BL21(DE3) Donor: Campylobacter spp.a, Neisseria spp.b, Bibersteinia spp.c | Issued for public comments on October 24, 2024; |
Source: E. coli W (ATCC 9637) Donor: Saccharomyces cerevisiaea, Synechocystis spb, Rhodobacter capsulatusc, Pasteurellamultocidad, Neisseria lactamicae | |||||
Source: E. coli K-12 DH1 MDO Donor: Campylobacter spp.a, Neisseria spp.b | |||||
a, f, j are donors for N-acetylneuraminic acid synthase; a,j are donors for UDP-N-acetylglucosamine epimerase; b, g, j are donors for CMP-N-acetylneuraminic acid synthase; c, h, i are donors for α-2,3-sialyltransferase; d is the donor for glucosamine-6-phosphate N-acetyltransferase; e is the donor for N-acetylglucosamine-2-epimerase. | |||||
8 | 6’-fucosyllactose, 6’-FL | 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.14-0.40 g/L (count as 6’-FL, the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli W NEO6 Donor: Saccharomyces cerevisiaea, Synechocystis sp.b, Pasteurellamultocidac, Photobacterium damselaed, Rhodobacter capsulatuse | Issued for public comments on March 13, 2024; |
a is the donor for glucosamine-6-phosphate N-acetyltransferase; b is the donor for N-acetylglucosamine-2-epimerase; c is the donor for CMP-N-acetylneuraminic acid synthase; d is the donor for α-2,6-sialyltransferase; e is the donor for N-acetylneuraminic acid synthase. | |||||
9 | Lacto-N-neotetraose | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.2-0.6 g/L (count as the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source:E. coli BL21 star (DE3) Donor: Neisseria spp.a, Helicobacter spp.b | Issued for public comments on May 10, 2024; |
10 | source: E.coli BL21(DE3) Donor: Neisseria spp.a, Helicobacter spp.b | Issued for public comments on October 24, 2024; | |||
a is the donor for β-1,3-N-acetylglucosaminyltransferase; b is the donor for β-1,4-galactosyltransferase. | |||||
11 | Lacto-N-tetraose | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.23-1.82 g/L (count as the state of ready to eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, Lacto-N-neotetraose, galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of Lacto-N-tetraose shall not exceed 64.5 (g/kg). | source: star(DE3)E. coli BL21 star(DE3) Donor: Neisseria spp.a, , Salmonella spp.b | Issued for public comments on June 24, 2024; |
a is the donor for β-1,3-N-acetylglucosaminyltransferase; b is the donor for β-1,3-galactosyltransferase. | |||||
12 | β-alanine | 13.05 Other special dietary foods (excluding 13.01-13.04) (limited to sports nutrition foods) | 2-4g/day | / | Issued for public comments on September 26, 2024; |
13 | fructo-oligosaccharide (FOS) | The scope of use and dosage of this substance shall comply with the provisions for approved FOS in the National Food Safety Standard for the Use of Food Nutritional Fortifiers (GB 14880). | / | Issued for public comments on September 26, 2024; | |
2) List of new food additives issued for public comments (3 types)
S.N. | Name | Function | Category number | Food name/ category | Maximum levels (g/kg) | Approval status (As of the end of December 2024) |
1 | Hydroxytyrosol | Antioxidant | 02.01.01 | Vegetables oils and fats | 0.05 | 1) Issued for public comments on May 10, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. |
2 | Steviol glycosides (fermentation) | Sweetener | The scope of use and dosage of steviol glycosides (fermentation method) shall comply with the provisions of the National Food Safety Standard for the Use of Food Additives (GB2760-2024). It can be used alone or mixed with steviol glycosides and steviol glycosides (enzyme conversion method), calculated as steviol equivalent. | Issued for public comments on June 24, 2024; | ||
The production strain information for steviol glycosides (fermentation method) is as follows: Source: E. coli BL21(DE3) Donors: Taxuscanadensisa, Stevia rebaudianab,Arabidopsis thalianac, Oryza sativad a is the donor for geranylgeranyl pyrophosphate synthase; b is the donor for pyrophosphate synthase, ent-kaurene synthase, ent-kaurene oxidase, cytochrome P450 oxidoreductase enzyme, and glycosyltransferase; c is the donor for cytochrome monooxygenase; d is the donor for glycosyltransferase. | ||||||
3 | butylated hydroxytoluene (BHT) | Antioxidant | The scope of use and dosage of butylated hydroxytoluene (BHT) shall comply with the provisions of the National Food Safety Standard for the Use of Food Additives (GB2760). | Issued for public comments on August 14, 2024; | ||
3) List of new enzyme preparations for the food industry (9 types)
S.N. | Name | Source | Donor | Approval status (As of the end of December 2024) |
|---|---|---|---|---|
1 | D-allulose-3-epimerase | Bacillus subtilis | Ruminococcus sp. 5_1_39B_FAA | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. |
2 | Glucoamylase | Aspergillus niger | Penicillium oxalicum | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
3 | Serine protease | Fusarium venenatum | Fusarium oxysporum | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
4 | Lipase | Komagataella phaffi | Aspergillus oryzae | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
5 | Transglutaminase | Bacillus licheniformis | Streptomyces mobaraensis | Issued for public comments on September 26, 2024; |
6 | Phosphodiesterase I | Leptographium procerum | — | Issued for public comments on September 26, 2024; |
7 | Lipase | Komagataella phaffi | Streptomyces sp. | Issued for public comments on September 26, 2024; |
8 | Peroxidase | Aspergillus niger | Marasmius scorodonius | Issued for public comments on October 24, 2024; |
9 | Xylanase | Aspergillus niger | Rasamsoniaemersonii | Issued for public comments on October 24, 2024; |
4) List of new food processing aids issued for public comments (1 type)
S.N. | Name | Function | Applicable scope | Approval status |
|---|---|---|---|---|
1 | Dichloromethane | Extraction solvent | decaffeination process of tea ≤ 2 mg/kg | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. |
5) List of new food spices issued for public comments (3 types)
S.N. | Name | Function | Applicable scope | Approval status |
|---|---|---|---|---|
1 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | food spices | This applies to the food additive 4-hydroxy-2,5-dimethyl-3(2H)-furanone produced from rhamnose as the raw material. The remaining provisions comply with the National Food Safety Standard for Food Additive 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone (GB 28365). | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
2 | Synthetic Anethole | food spices | It can be formulated into food flavorings for use in various food categories (excluding those listed in Table B.1 of GB 2760-2014) and used in appropriate amounts as needed for production. | Issued for public comments on September 26, 2024; |
3 | Hydroxycitronellal | food spices | It can be formulated into food flavorings for use in various food categories (excluding those listed in Table B.1 of GB 2760-2014) and used in appropriate amounts as needed for production. | Issued for public comments on September 26, 2024; |
6) Food nutrition enhancer with expanded scope (3 types)
S.N. | Name | Category No. | Food name | Applicable scope | Approval status |
|---|---|---|---|---|---|
1 | (6S)-5-methyltetrahydrofolate, glucosamine salt | 13.05 | Other special dietary foods, except for categories 13.01 to 13.04 (limited to nutritional supplements for pregnant and lactating women), | It shall comply with the folic acid requirements specified in the National Food Safety Standard for Nutritional Supplements for Pregnant and Lactating Women (GB 31601). | 1) Issued for public comments on March 13, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
2 | Sodium ferrous citrate | As a source of iron compounds, the scope of use and dosage shall comply with the provisions of the National Food Safety Standard for the Use of Food Nutrition Enhancer (GB 14880) regarding iron. | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | ||
3 | 2'-fucosyllactose, 2'-FL | 13.02.01 | Infant cereal-based complementary foods | 0.7-2.4 g/L (calculated as pure product, in ready-to-eat state; for powdery products, the level of use should be increased by times of brewing), when mixed with 2’-fucosyllactose, Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of these substances should not exceed 64.5 g/kg. | Issued for public comments on September 26, 2024; |
13.02.02 | Infant complementary feeding products | ||||
7) List of food additives with expanded scope issued for public comments (8 types)
S.N. | Name | Function | Category number | Food name/ category | Maximum levels (g/kg) | Remarks | Approval status (By the end of December 2024) |
|---|---|---|---|---|---|---|---|
1 | Polyglyceryl ricinoleate | Emulsifier | 01.05.03 | Pickled vegetables | 10.0 | - | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. |
2 | Sodium cyclamate | Sweetener | 01.02.02 | Flavored fermented milk | 0.65 | Calculated as cyclohexylamine sulfonic acid | 1) Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
16.07 | Others (limited to konjac gel products) | 1.0 | |||||
3 | Steviol glycosides | Sweetener | 01.03.02 | Formulated milk powder and formulated cream powder | 0.3 | calculated as steviol equivalent | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
01.06.04 | Processed cheese and cheese products | 0.4 | |||||
04.03.02.03 | Pickled edible fungi and algae | 0.23 | |||||
06.07 | Instant rice and noodle products | 0.4 | |||||
4 | Curdlan gum | Thickener, stabilizer and coagulant | 01.05.03 | Formulated cream | Used in appropriate amounts as needed for production. | - | Issued for public comments on August 14, 2024; |
16.05 | Starter cultures for food processing (excluding 16.04) | ||||||
5 | Steviol glycosides (enzymatically converted) | Sweetener | The scope of use and dosage shall comply with the provisions for approved steviol glycosides in the National Food Safety Standard for the Use of Food Additives (GB 2760-2024). It can be used alone or in combination with other steviol glycosides. | calculated as steviol equivalent | Issued for public comments on August 14, 2024; | ||
6 | Sucralose | Sweetener | 04.01.02.08 | preserved fruits | 2.8 | - | Issued for public comments on September 26, 2024; |
7 | Acesulfame Potassium | Sweetener | 14.08 | Flavored beverages | 0.5 | -in ready-to-eat state; for powdery products, the level of use should be increased by times of brewing | Issued for public comments on September 26, 2024; |
8 | Magnesium Oxide | Thickener | 04.01.01.02 | Surface-treated fresh fruits (limited to citrus fruits) | Used in appropriate amounts as needed for production. | - | Issued for public comments on October 24, 2024; |
8) List of processing aids with expanded scope and use issued for public comments (1 type)
S.N. | Name | Function | Scope of use | Approval status (By the end of December 2024) |
|---|---|---|---|---|
1 | Hydrogen peroxide | Desulfurizer | Yeast-derived product processing technology | Issued for public comments on October 24, 2024; |
4. List of approved new food additives in 2024 (30 types)
In 2024, 30 new food additives were approved by NHC, including 4 new food additives, and 8 food additives with expanded use and scope. Detailed information is as follows:
1) List of new food nutrition enhancers (8 types)
S.N. | Name | Source | Donor | Acceptance information, issuance for public comments and approval (As of the end of December 2024) | ||||
|---|---|---|---|---|---|---|---|---|
1 | 2’-fucosyllactose, 2’-FL | E. coli BL21(DE3) | Helicobacter pyloria | 1) Issued for public comments on October 26, 2023; 2) Officially approved on March 13, 2024 according to No. 2 of 2024. | ||||
2 | 2’-fucosyllactose, 2’-FL | E. coli BL21 star (DE3) | Escherichia coli O126a | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. | ||||
3 | 2’-fucosyllactose, 2’-FL | Corynebacterium glutamicum ATCC 13032 | Pseudopedobacter saltansa | 1) Issued for public comments on August 23, 2023; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. | ||||
4 | 2’-fucosyllactose, 2’-FL | E. coli K-12 G GI724 | Bacteroides vulgatusa | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | ||||
5 | 2’-fucosyllactose, 2’-FL | E. coli BL21(DE3) | Bacteroidesa | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. | ||||
6 | 2’-fucosyllactose, 2’-FL | E. coli BL21 star (DE3) | Escherichia spp.a | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. | ||||
In the above steps 1-6, a is the donor for α-1,2-fucosyltransferase; The usage amount, scope, and quality specifications of 2'-fucosyllactose shall comply with the requirements of the 2023 No. 8 Announcement of the National Health Commission (excluding the production strain information for 2'-fucosyllactose in Appendix C). | ||||||||
7 | Lacto-N-neotetraose | E. coli BL21 star (DE3) | Neisseria spp.a, Helicobacter spp.b | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | ||||
a is the donor for β-1,3-N-acetylglucosamine transferase, and b is the donor for β-1,4-galactosyltransferase. The usage scope, amount, and quality specifications of lactose-N-neotetrose shall comply with the requirements of the 2023 No. 8 Announcement of the National Health Commission (excluding the production strain information for lactose-N-neotetrose in Appendix C). | ||||||||
S.N. | Name | Category No. | Food name | Applicable scope | Approval status | |||
8 | d-ribose | 13.05 | Other special dietary foods, excluding categories 13.01-13.04 (limited to sports nutrition foods) | 1~2 g/day | 1) Issued for public comments o 2021.10.21 2) Officially approved on March 13, 2024 according to No. 2 of 2024. | |||
2) List of approved new food additives (4 types)
S.N. | Name | Function | Category number | Food name/category | Maximum levels (g/kg) | Remarks | Acceptance information, issuance for public comments and approval (As of the end of December 2024) |
|---|---|---|---|---|---|---|---|
1 | Mixed tocotrienols concentrate | Antioxidant | 02.01.01 | Plant oils and fats | 0.2 | Calculated as total tocopherols and total tocotrienols |
|
2 | Steviol glycosides (enzymatic conversion method) | sweetener | 01.01.03 | Formulated milk | 0.18 | Can be used alone or mixed with steviol glycosides, calculated as steviol equivalent. |
|
01.02.02 | Flavored fermented milk | 0.2 | |||||
03.01 | Ice cream and ice lolly | 0.5 | |||||
05.02.01 | Gum-based confectionery | 3.5 | |||||
14.0 | Beverages [Excluding 14.01 Packaged drinking water, 14.02.01 Fruit and vegetable juices (Puree), and 14.02.02 Concentrated fruit and vegetable Juices (Puree)] | 0.2 | Can be used alone or mixed with steviol glycosides, calculated as steviol equivalent; for ready-to-drink products, the usage for corresponding solid beverages should be increased proportionally based on the dilution ratio. | ||||
The production strain information for steviol glycosides (enzymatic conversion method) is as follows: Source: E. coli BL21 (DE3); Donors: Methylocaldum szegediensea, Stevia rebaudiana Bertonib, and Solanum tuberosumc. a is the donor for sucrose synthase; b is the donor for β-1,3-glycosyltransferase; c is the donor for β-1,2-glycosyltransferase. | |||||||
3 | Hydroxytyrosol | Antioxidant | 02.01.01 | Plant oils and fats | 0.05 | — | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. |
4 | Dichloromethane | Extraction solvent | decaffeination process of tea ≤ 2 mg/kg | — | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. | ||
3) New enzyme preparations for the food industry (7 types)
S.N. | Name | Source | Donor | Approval status (As of the end of December 2024) |
|---|---|---|---|---|
1 | D-psicose 3-epimerase | Bacillus subtilis | Clostridium scindens ATCC35704 | 1) Issued for public comments on December 29, 2023; 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
2 | Cyclodextrin glucanotransferase | Anoxybacillus caldiproteolyticus | — | 1) Issued for public comments on 2023.10.26. 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
3 | Cellulase | Penicillium oxalicum | — | 1) Issued for public comments on 2023.10.26. 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
4 | D-psicose 3-epimerase | Bacillus subtilis | Ruminococcus sp.5_1_39B_FAA | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. |
5 | Glucoamylase | Aspergillus niger | Penicillium oxalicum | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
6 | Serine protease | Fusarium venenatum | Fusarium oxysporum | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
7 | Lipase | Komagataella phaffi | Aspergillus oryza | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
Note: The quality specifications of food enzymes shall meet the requirements specified in National Food Safety Standard - Food Additives and Enzymes (GB 1886.174).
4) List of new spice for food (1 type)
S.N. | Name | Food name | Maximum level (g/L) | Remark | Acceptance information, issuance for public comments and approval (As of the end of December 2024) |
1 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | Spice for food | Used in appropriate amounts in production | — | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
5) List of approved new food additives with expanded scope and use (8 types)
S.N. | Name | Function | Category number | Food name/category | Maximum levels (g/kg) | Remark | Acceptance information, issuance for public comments and approval (As of the end of December 2024) |
|---|---|---|---|---|---|---|---|
1 | Propylene glycol alginate | Thickener | 06.05.02.01 | Vermicelli, noodles | 1.5 | — | 1) Issued for public comments on Issued for public comments on 2023.08.23 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
06.05.02.04 | Tapioca pearls | — | |||||
2 | Polyoxyethylene (20) sorbitan monooleate (also known as Tween 80) | Emulsifier | 16.03 | Collagen sausage casings | 0.5 | — | 1) Issued for public comments on Issued for public comments on 2023.10.26 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
3 | Ascorbyl palmitate (enzymatic) | Antioxidant | 01.03.02 | Formulated milk powder and formulated cream powder | 0.2 | Calculated as ascorbic acid in fat | 1) Issued for public comments on Issued for public comments on 2023.12.29 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
07.01 | Bread | 0.2 | — | ||||
14.05.01 | Tea beverage | 0.2 | For ready-to-drink products, the usage for corresponding solid beverages should be increased proportionally based on the dilution ratio. | ||||
4 | Rosemary extract | Antioxidant | 04.05.02 | Flour pastes (e.g., drag flour pastes used for fish and poultry), breading and frying flour | 0.3 | — | 1) Issued for public comments on Issued for public comments on 2023.10.26 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
5 | Sucralose (also known as Splenda) | Sweetener | 04.05.02.01.01 | Instant rice flour products | 4.0 | — | 1) Issued for public comments on Issued for public comments on 2023.12.29 2) Officially approved on March 13, 2024 according to No. 2 of 2024. |
04.05.02.01.02 | 2.0 | ||||||
6 | Polyglycerol polyricinoleate | Emulsifier | 01.05.03 | Formulated whipping cream | 10.0 | — | 1) Issued for public comments on March 13, 2024; 2) Officially approved on August 5, 2024 according to No. 3 of 2024. |
7 | Sodium cyclamate | Sweetener | 01.02.02 | Flavored fermented milk | 0.65 | Calculated as cyclohexylamine sulfate | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
16.07 | Others (only konjac gel products) | 1.0 | |||||
8 | Steviol glycosides | Sweetener | 01.03.02 | Formulated milk powder and formulated cream powder | 0.3 | Calculated as steviol equivalent | 1) Issued for public comments on Issued for public comments on June 24, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
01.06.04 | Processed cheese and cheese products | 0.4 | |||||
04.03.02.03 | Pickled edible mushrooms and algae | 0.23 | |||||
06.07 | Instant rice and noodle products | 0.4 |
6) List of approved food nutrition enhancers with expanded scope and use (2 types)
S.N. | Name | Category number | Food name | Maximum levels | Approval status (By the end of December 2024) |
|---|---|---|---|---|---|
1 | Sodium ferrous citrate | As a source of iron compound, the scope of use and dosage shall comply with the regulations on iron in the National Food Safety Standard for Food Nutrition enhancers (GB 14880). | 1) Issued for public comments on May 10, 2024; 2) Officially approved on October 10, 2024 according to No. 5 of 2024. | ||
2 | (6S)-5-Methyl tetrahydrofolate, glucosamine salt | 13.05 | Other special dietary foods excluding 13.01–13.04 (only for nutritional supplements for pregnant women and lactating mothers) | Calculated as folic acid, in accordance with the regulations on folic acid in the National Food Safety Standard for Nutritional Supplements for Pregnant Women and Lactating Mothers (GB 31601). | 1) Issued for public comments on March 13, 2024; 2) Officially approved on December 13, 2024 according to Notice No. 6 of 2024. |
CIRS opinion
In 2024, the number of new food additives received and soliciting opinions has increased compared to 2023, while the number of approved products remained largely the same as in 2023. It is noteworthy that most of the approved products this year are HMOs, with many domestic applicants. This indicates that the industrialization of HMO technology is no longer a bottleneck issue for China, and the Chinese market for HMOs is now entering a very broad development space. 6 varieties of 2’-FL and one of LNnT have been approved and announced, along with several 2’-FL products from different microbial sources, as well as 3-FL, LNT, LNnT, 3’-SL, and 6’-SL, all of which have passed official technical reviews, with public opinion drafts being released. Overall, with the increasing number of applications, the official review speed has noticeably accelerated. However, in the case of HMO, the number of applications and approvals are not proportional, so companies need to make adequate preparations regarding product safety and the necessity of the technology used, submitting comprehensive documentation to prepare for the evaluation process.
For changes in the acceptance, public consultations, approval announcements, and other application information about new food additive varieties, you can inquire the ChinafoodDB, a one-stop free digital intelligent query platform for food ingredients and additives under the CIRS Group. Know more details about the ChinafoodDB - Announcing the Launch of ChinaFoodDB – The Free Platform for Searching China Food and Health Food Ingredients!
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

