Updated on January 8, 2024
On August 31, 2023, the State Administration for Market Regulation (SAMR) issued an announcement regarding the Directory of Health Functions Available to Be Claimed by Health Food - Non-nutrition Supplements (2023 Version) and the supporting documents. According to the announcement, products with registration certificates marked as “No expiry date and product technical requirements” (hereinafter referred to as “dual-no” products) are required to complete the certificate renewal process in accordance with current laws and regulations within a 5-year transition period. On November 1, 2024, the SAMR issued the Key Points for Review of Health Food In-Production and On-Sale with “No Expiry Date and No Technical Requirements”, aimed at standardizing the registration certificates of “dual-no” products that are currently in production or being sold.
Related Links
“Dual-no” products that need to redo or supplement function tests
According to the latest regulations, registered products that fall in both of the following categories are required to redo or supplement function tests:
- The function evaluation was done based on the Health Food Function Evaluation Procedures and Methods (1996 Version); and
- Product functions fall within the eight health function categories listed in Table 1.
Table 1. Eight health function categories that require new or supplementary function tests
S.N. | Previous health functions | New health functions | Function tests that need to be redone when the evaluation was done based on the Health Food Function Evaluation Procedures and Methods (1996 Version) |
1 | Regulating immunity; enhancing immunity | Aids in enhancing immunity | Redo animal function tests |
2 | Delaying aging; anti-oxidation | Aids in anti-oxidation | Supplement human feeding trials |
3 | Improving memory; aids in improving memory | Aids in improving memory | Redo human feeding trials if the Wechsler memory scale is used for the trials. |
4 | Anti-fatigue; alleviating physical fatigue | Alleviating physical fatigue | Redo animal function tests if pole test is used for motor function testing. |
5 | Tolerant to hypoxia; enhancing hypoxia tolerance | Tolerant to hypoxia | Redo animal function tests |
6 | Losing weight | Aids in controlling body fats | Redo functional tests |
7 | Regulating blood lipids (lowering total cholesterol and reducing triglycerides); assisting in lowering blood lipids | Aids in maintaining healthy blood lipid (cholesterol/triglyceride) levels | Redo human feeding trials |
8 | Radiation resistance; providing auxiliary protective function against radiation hazards | Provides auxiliary protective action against ionizing radiation hazards | Redo animal function tests |
Certificate renewal process
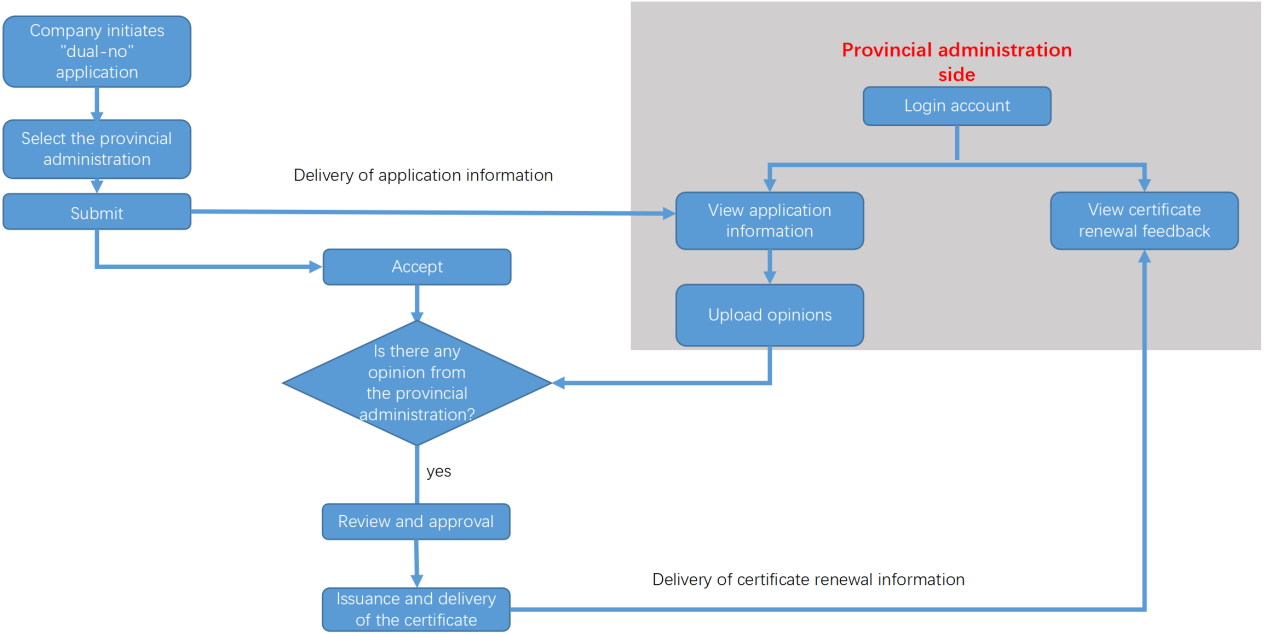
Note: For imported “dual-no” products, it is not required to obtain a certificate renewal opinion from the provincial administrations.
It is recommended that applicants submit a request to the provincial administrations that are responsible for the issuance of production licenses for the “certificate renewal opinion” before initiating the application for the certificate renewal for the “dual-no” products, to ensure that the submitted documents align with the attachments mentioned in the renewal opinion (including formulas, production process, and product technical requirements).
The certificate renewal processes may vary in different provincial administrations. In Guangdong Province, the workflow is as follows:
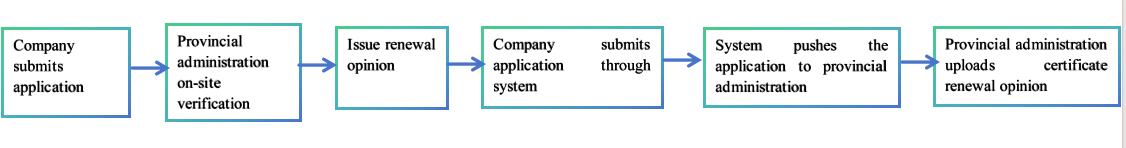
Note: Please consult the local provincial administrations for their renewal policies.
Certificate renewal requirements
Domestic products | Imported products |
1) Application form and the applicant’s legal responsibility commitment letter regarding the authenticity of the application materials | 1) Application form and the applicant’s legal responsibility commitment letter regarding the authenticity of the application materials |
2) A copy of the valid registration proof document of all registrants | 2) A copy of the valid registration proof document of all registrants |
3) A copy of the health food approval certificate and related change, transfer, etc. approval documents | 3) A copy of the health food approval certificate and related change, transfer, etc. approval documents |
4) Details of the changes, reasons, and basis | 4) Qualification certification documents issued by the competent authorities or legal service institutions in the product’s country (region) of production proving that the registrant is the overseas manufacturer of the listed health food |
In addition to the above materials, the following materials are also required for certificate renewal of "dual-no" products: | 5) Health food sales approval documents issued by the competent authorities or legal service institutions in the product’s country (region) of production |
1) The status of valid production licenses nationwide (listing the provincial-level market supervision departments issuing the production licenses, SC number, issue date, and expiration date) | 6) The registrant (overseas manufacturer)’s filing or registration information with the inspection and quarantine authorities in China, along with the product’s import information |
2) A commitment letter from the registrant stating that there are no legal disputes or ownership disputes over the product registration certificate, and that the product involved in the certificate renewal is not under any unresolved law enforcement cases | 7) Original technical regulations or standards related to health foods issued by the product’s country (region) of production or international organizations |
3) Application for product name change or retention of the product name, and a copy of the trademark registration certificate (no need to provide if there is no registered trademark) | 8) If the registration matters are handled by the foreign registrant’s permanent representative office in China, submit the Registration Certificate for the Permanent Representative Office of Foreign Enterprises in China and its copy; if a domestic agency is entrusted by the foreign registrant, submit the notarized original power of attorney and a copy of the business license of the entrusted agency |
4) Product technical requirements drafted in accordance with current regulations, and materials such as revision explanations, research materials, test reports, etc. | 9) Details of the changes, reasons, and basis |
5) Revised draft of the product label and the revision explanation | In addition to the above materials, the following additional materials are also required for certificate renewal of "dual-no" products: |
6) Post-market safety evaluation report on consumer safety | 1) Application for product name change or retention of the product name, and a copy of the trademark registration certificate (no need to provide if there is no registered trademark) |
7) Necessary functional test reports, legal source proof for raw and auxiliary materials, and other application materials | 2)A commitment letter from the registrant stating that there are no legal disputes or ownership disputes over the product registration certificate, and that the product involved in the certificate renewal is not under any unresolved law enforcement cases |
/ | 3) Two samples of the smallest sales packaging for overseas sales or imported sales, along with the revised draft of the product label and revision explanation |
/ | 4) Post-market safety evaluation report on consumer safety |
/ | 5) Product technical requirements drafted in accordance with current regulations, along with revision explanations, research materials, test reports, etc. |
/ | 6) Necessary functional test reports, legal source proof for raw and auxiliary materials, and other application materials |
Estimated registration period
Generally, it takes about 1-3 years to complete the renewal of registration certificate (From dossier preparation to government review and approval).
Our services
- Health Food Formula and Production Process Research and Development
- Domestic/Imported Health Food Registration
- Domestic/Imported Health Food Filing
- Domestic/Imported Health Food Registration Renewal
- Domestic/Imported Health Food Technology Transfer Registration
- New Health Food Functions Application
Further Information
FAQs on the Replacement of Health Food Registration Certificate in China (Vol. 1)
China Health Food Registration and Filing
Registration Status of Health Food in China in the first half of 2023
Filing Status of Chinese Health Foods (Dietary Supplements) in the First Half of 2023